Abstract
Bronchiectasis is a chronic progressive disease characterized by irreversible pathological dilation of pulmonary bronchi. Treatments for bronchiectasis are aimed at mobilizing airway secretions, reducing inflammation, preventing respiratory infections, enhancing ventilation, minimizing the number of exacerbations, and improving a person’s quality of life. High frequency chest wall oscillation (HFCWO) is an airway clearance treatment currently used for a number of chronic airway compromising diseases including non-cystic fibrosis bronchiectasis. This study evaluated the economic impact of HFCWO treatment delivered by the SmartVest® Airway Clearance System on bronchiectasis-related healthcare utilization and cost.
Methods
The results of a previously published case review outcome-based clinical study by the authors provided the basis for this cost effectiveness analysis. Bronchiectasis-related exacerbations including the number of hospitalizations, emergency department (ED) visits and frequency of antibiotic prescriptions were recorded for each patient for a one year period prior to SmartVest use (standard of care control) and for a one year period after starting SmartVest use. The exacerbation rates for one year pre-SmartVest and one year post-SmartVest were compared. Exacerbations were verified from both the patient’s medical records and by phone interview. Antibiotic costs were determined using “on-line discount pharmacy pricing” whereas hospitalization and ED costs were determined using the Healthcare Cost and Utilization Project (HCUP) Statistical Brief #146 and the HCUP National Inpatient Sample (NIS) 2013 database.
Results
The previously published clinical outcomes of fifty-nine SmartVest patients with non-cystic fibrosis bronchiectasis served as the basis for this analysis. When the outcome data were analyzed, SmartVest use, compared to the standard of care control, was associated with statistically significant results; a 58% decrease in antibiotic cost, a 63% decrease in ED visit cost and a 60% decrease in hospitalization cost. In total, the cost analysis resulted in an annual savings of $3,045 per patient per year of SmartVest use.
Conclusions
The clinical effectiveness of using SmartVest as a treatment for non-cystic fibrosis bronchiectasis patients was previously verified by a significant reduction in bronchiectasis-related exacerbations, which directly translates into a significant 60% overall reduction in healthcare utilization and cost in this population. Furthermore, secondary benefits such as the potential to reduce hospital readmissions and the potential to impact in deterring antibiotic resistance may have even greater benefits than decreasing cost.
Keywords
SmartVest, high frequency chest wall oscillation, HFCWO, bronchiectasis, cost
Introduction
Bronchiectasis is a chronic and etiologically heterogeneous disease. Common characteristics of bronchiectasis are shortness of breath, frequent exacerbations, chronic cough, hemoptysis, and excessive sputum production. The disease is typically characterized by cycles of impaired mucociliary clearance, bronchial infection, and inflammation resulting in structural damage to the airways with permanent and abnormal dilation.1 Bronchiectasis can be the outcome from a diverse array of respiratory and systemic diseases, including cystic fibrosis, dyskinetic ciliary syndromes, inhalation/aspiration injuries, primary and acquired immunodeficiency states, and a number of rheumatic and inflammatory conditions.2 Bronchiectasis is observed in 7% to 52% of patients with asthma or chronic obstructive pulmonary disease (COPD).3,4,37
Seitz, et al, analyzed a 5% sample of the Medicare outpatient claims database for bronchiectasis among beneficiaries aged ≥65 years from 2000 to 2007.6 The database contains claims-level information from non-institutional outpatient healthcare providers. Bronchiectasis was identified by the database using the International Classification of Diseases, Ninth Revision, Clinical Modification codes (ICD-9-CM) codes. The study population included >2 million unique individuals enrolled in Medicare Part B for at least one month from 2000 to 2007. The study determined the prevalence of bronchiectasis in the overall population to be 1,106 cases per 100,000 people over the eight-year review period. The study also found that the prevalence of bronchiectasis in Medicare beneficiaries increased by 8.7% between 2000 and 2007 and the hospitalization rate for bronchiectasis increased annually at a rate of 2.4% among men and 3.0% among women.
The overall burden of advanced lung disease is rising, and where data exist, the costs related to the morbidity and mortality of these diseases appear significant.2 This might, in part, be a reflection of the increasing aging population with chronic lung disease which has a disproportionate rise in health-care costs; the rate of hospitalization due to chronic lung disease markedly increases above the age of 50 years, and particularly in older women.2,7 Using discharge records from between 1997-2010, it was estimated the mean hospital cost for inpatient care in patients with a pneumonia exacerbation was $9,300.28 In 2001, it was also estimated that the annual medical cost of care for persons in the United States with bronchiectasis was $13,244, which is greater than the annual cost for many other chronic diseases, such as heart disease ($12,000) and COPD ($11,000).38 A 2005 study found that patients with non-cystic fibrosis bronchiectasis averaged 2.0 additional days per year in the hospital, had 6.1 additional outpatient encounters and 27.2 more days of antibiotic therapy compared with patients without the disease.9 In 2005, the treatment costs for non-cystic fibrosis bronchiectasis was $630 million annually.5
Patients with non-cystic fibrosis bronchiectasis can have difficulty clearing airway secretions and can significantly benefit from airway clearance therapy.2 The aims of treatment for bronchiectasis are to mobilize airway secretions so as to reduce inflammation, prevent respiratory infections, enhance ventilation, minimize the number of exacerbations, and improve a patient’s quality of life.10-12 A number of therapeutic methods are currently used to clear airway secretions in patients with pulmonary disease, respiratory mucus clearance impairment, or who are at risk of developing either one of those conditions.8 These methods generally aim to promote secretion clearance by reducing mucus viscosity and using shear forces to release the mucus from the lung wall to facilitate mobilization for ease of expectoration. Standard of care involves combination therapy with mucolytic and mucokinetic agents, bronchodilators, anti-inflammatory therapy, and some form of physical/mechanical airway clearance therapy.2 Airway clearance therapy plays a critical role as it helps to avoid retention of pathogen-laden mucus which is the underlying origin of recurrent infection that causes progressive pulmonary deterioration.2,13,14 Airway clearance methods play a critical role in maintaining respiratory health throughout the life-time of the patient.
A number of airway clearance methods are available including chest physiotherapy, positive end-expiratory pressure masks, oral-high frequency devices, and high frequency chest wall oscillation (HFCWO).2 Positive end-expiratory pressure masks and oral-high frequency devices require active effort, mastery of the technique, and/or physical agility which can limit their use.2
HFCWO is used for airway clearance in patients with a wide range of airway compromising diseases and conditions, including genetic and immunological disorders, neuromuscular diseases, and obstructive pulmonary conditions, such as asthma and COPD.15-18 In contrast to some other methods, HFCWO requires minimal activity from the user and is not dependent on a Healthcare Provider’s technique to be effective.2 Clinical studies, primarily in patients with cystic fibrosis, have shown HFCWO to be safe and effective.2,15,19-22 HFCWO delivers compression pulses to the chest wall through an inflatable vest connected to an air pulse generator.2 The generator produces an alternating flow of air into, and out of, the vest that rapidly compresses and releases the chest wall within a range of selectable frequencies and pressures. The oscillatory compression imparted to the chest wall has been reported to thin viscous mucus, disconnect mucus from the lung’s wall, and propel mucus from the minor airways of the lungs toward the major airways where it can be expectorated or suctioned away.2,23,24 HFCWO can lead to significant improvement in lung volume of 15 to 57mL and in flow up to 1.6L/sec.22
A prior case review study evaluated the clinical outcomes of SmartVest® Airway Clearance System therapy on exacerbation-related healthcare utilization and medication use in subjects with non-cystic fibrosis bronchiectasis.25 The study found that the use of SmartVest resulted in a statistically significant 60% reduction in bronchiectasis-related exacerbations including antibiotic use, emergency department (ED) visits and hospitalizations. The current study is designed to assess the economic impact of SmartVest therapy on bronchiectasis-related healthcare and antibiotic costs.
Methods
A recent SmartVest (Electromed, Inc., New Prague, MN, USA) case review outcome-based clinical study served as the basis for this analysis.25 The study recorded all bronchiectasis-related exacerbations to include the number of hospitalizations, ED visits and antibiotic use for a one year period prior to SmartVest use (standard of care control) and, for comparison, for a one year period after the start of SmartVest use (treatment). The study included only those subjects with a diagnosis of non-cystic fibrosis bronchiectasis who had been using SmartVest for at least one year, and whose medical records were available for one year prior to initiation of SmartVest therapy. Patients were excluded if they had not been compliant with their prescribed SmartVest therapy regimen, were unable to be contacted by phone, or had expired. All data collected prior to SmartVest use were captured via the patient’s medical records.
Patient’s medical records were reviewed for all bronchiectasis-related exacerbations that occurred during a one year period prior to starting SmartVest therapy. Subjects were contacted and interviewed by phone to collect bronchiectasis-related exacerbations for the one year period after starting SmartVest therapy. The questionnaire for the phone survey was developed for the exclusive purpose of the study. During the phone interview, the subject was asked specific questions regarding respiratory-related antibiotic use, ED visits and hospitalizations. The interview also inquired whether the patient was using SmartVest according to the physician’s prescription regimen.
Antibiotic costs were determined using “on-line discount pharmacy pricing” however, the cost of office visits and physician fees were not included. Hospitalization and ED costs were determined using the weighted national estimates from Healthcare Cost and Utilization Project (HCUP) National Inpatient Sample (NIS), 2013, Agency for Healthcare Research and Quality (AHRQ), based on data collected by individual States and provided to AHRQ by the States.26 NIS database includes a stratified probability sample of hospitals from State Inpatient Databases that include hospitalizations by patients with Medicare, Medicaid, private insurance, and the uninsured.27 The NIS contains data from 5 to 8 million hospital stays from about 1000 hospitals. It is designated to approximate a 20% sample of the US nonfederal, short-term hospitals as defined by the American Medical Association. The NIS is drawn from states participating in the Healthcare Cost and Utilization Project. The NIS contains uniform inpatient stay data from hospital discharge databases maintained by state agencies, hospital associations, and other private organizations. Total number of weighted discharges in the US based on HCUP NIS was 35,597,792.28
Results
Review of HCUP and Medicare databases revealed associated healthcare costs for a bronchiectasis-related exacerbation
to be $450 (2012) for an ED visit and $9,300 (2010) for a hospitalization with pneumonia. Extended hospital stays based on complications or comorbidities were not calculated. Using on line discount pharmacy pricing calculations, the cost of a standard antibiotic regimen for pneumonia was $290 (2016). Physician fees for an office visit and subsequent prescription were not available for calculation.
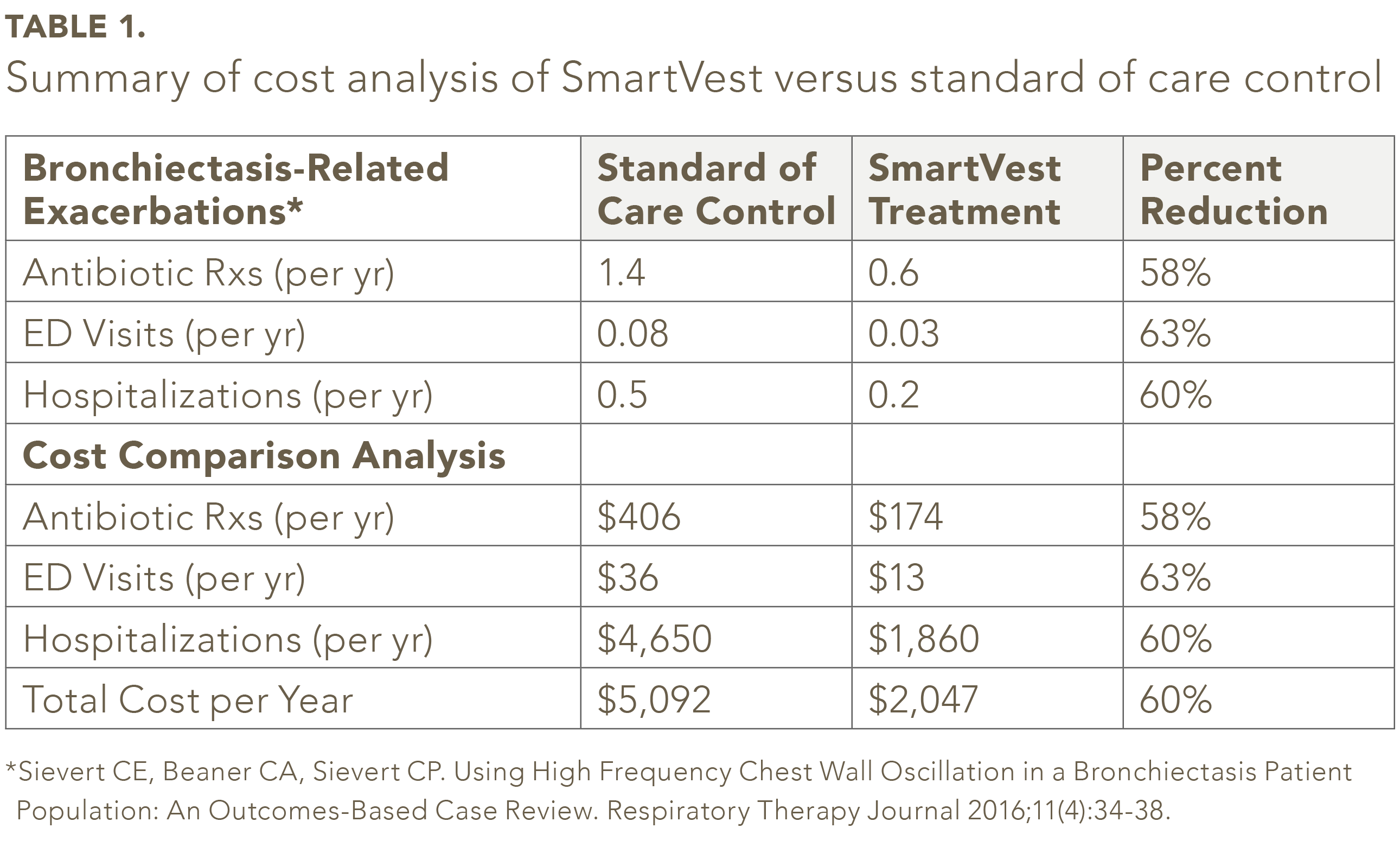
Of the 104 bronchiectasis SmartVest patients identified, fifty-nine patients met the inclusion/exclusion criteria.25 In the study population, the average number of antibiotic prescriptions per year was 58% less for SmartVest (0.6/yr) compared to standard of care control (1.4/yr) (see Table 1). SmartVest use also significantly reduced ED visits by 63% (0.08/yr verses 0.03/yr) and hospitalizations by 60% (0.5/yr verses 0.2/yr). The annual per patient costs for antibiotics for patients treated with SmartVest were about $233 lower compared to those treated with standard of care (see Table 1). Hospitalizations, after one-year of SmartVest use, were also significantly reduced by $2,790 per patient per year. In total, the overall results revealed an annual savings of $3,045 per patient per year of SmartVest use.
The analysis did not account for any physician/office fees incurred to obtain an antibiotic prescription without hospitalization. Also, the analysis did not account for an office visit for a potential exacerbation that did not result in a prescription or hospitalization. In addition, the analysis did not account for added expense if the exacerbation was an antibiotic resistant bacterial strain pneumonia which reportedly can cost more than $15,000 for each episode.
Discussion
The purpose of the analysis was to evaluate the economic impact of SmartVest use on bronchiectasis-associated medical costs compared to a standard of care control. To our knowledge, this is the first study to assess the healthcare costs of treating non-cystic fibrosis bronchiectasis patients with HFCWO. Overall cost included the cost of antibiotics, ED visits, and hospitalizations related to exacerbations associated with non-cystic fibrosis bronchiectasis. The study demonstrated a significant reduction in healthcare utilization and its associated cost when bronchiectasis patients were treated with SmartVest for one-year. The overall cost was reduced by 60% which translates into a savings of $3,045 per patient per year.
A recent study that evaluated hospital discharges, readmissions, and ED visits for COPD or bronchiectasis in adults in the United States found from 2001 to 2012 the number of hospital discharges rose by 88,000.31 The study also found that about 7% of patients with COPD or bronchiectasis were readmitted within 30 days with COPD or bronchiectasis as the principle diagnosis.31 In contrast, the rate of discharge decreased for other diseases.31 The reason for the significant rise in COPD and bronchiectasis hospital and ED visits is not clear as a significant decline in rates of smoking have been observed. However, it may reflect the potential under diagnosis of the disease and the long-term nature of COPD and bronchiectasis in an aging population.31
A previous case review outcome-based study reported that SmartVest use reduced hospitalizations by 1.5 fold,25 indicating the cost benefit of HFCWO on reducing healthcare utilization burden. Other studies have also evaluated factors (both system and patient) that may lower readmission in patients with COPD, and are, at least in part, relevant to patients with bronchiectasis. These factors include continuity with the patients’ primary care provided or pulmonologist, discharges coordinator intervention, and the extent or type of respiratory therapy.32-34
Reduction in the number of bronchiectasis-related exacerbations can also impact a patient’s quality of life.35,36 In a population of patients with COPD, the use of SmartVest was associated with significant improvement in the five-symptom score P=0.002 (rating of sputum, wheeze, cough, shortness of breath, and exercise tolerance).36 SmartVest treatment also demonstrated a significant improvement in the St. George’s Respiratory Questionnaire (SGRQ) P=0.02, while no improvement was observed in patients treated with conventional treatment.36 Similarly, our prior case review outcome-based study found that 68% of the subjects indicated during the phone call interview that the use of SmartVest had significantly improved their quality of life.25
Several limitations to the study design should be considered when interpreting the results. The patient size of the study was small, and the hospitalizations, ED visits, and antibiotic use data after initiation of SmartVest therapy was obtained primarily through patient interview. The study may be considered conservative due to no costing added for physician’s fees associated an office visit resulting in an antibiotic prescription or, no costing added for an office visit that did not result in an antibiotic prescription. In addition, Reliance on HCUP, NIS and AHRQ data bases, which depend on the diagnoses entered on claims, may be coded incorrectly or not coded at all, thereby potentially introducing measurement error with respect to ICD-9-CM-based variables.
Reducing healthcare utilization cost such as antibiotic use, ED visits and hospitalizations are prioritized objectives of recent healthcare directives such as the Affordable Care Act (ACA). For example, the ACA has established the Hospital Readmissions Reduction Program (HRRP), which has directed CMS to penalize hospitals by reducing reimbursement payments for excess patient readmissions for the same condition. HRRP originally identified the top three “applicable conditions” for focused readmission measurement to include acute myocardial infarction, heart failure and pneumonia. In addition, CMS recently finalized the expansion of additional applicable conditions beginning with the fiscal year 2015 program to include patients readmitted for an acute exacerbation of COPD. The significant reduction in healthcare utilization and hospitalizations for non-cystic bronchiectasis patients using SmartVest, as demonstrated in this study and others, may play a critical role in helping hospitals reduce readmissions and thus not be penalized.
For patients who have airway infections resistant to oral antibiotics, the burden is much greater and more serious. Intravenous antibiotics complicate care greatly because hospitalization or home monitoring is required. Treatment for these patients includes placement of a central venous catheter, coordination of the doses of drugs that often must be given multiple times per day, regular blood tests to monitor for side effects, and measurement of blood levels of the antibiotic for many days, steps that become expensive and disrupt patients’ lives.
The World Health Organization (WHO) has declared that microbial resistance to antibiotics poses a “major global threat with devastating implications to public health.” Antimicrobial resistance threatens the effective prevention and treatment of an ever-increasing range of infections caused by not only bacteria but viruses and fungi as well. The US Centers for Disease Control and Prevention (CDC) considers antimicrobial resistance one of their top concerns and priorities. In the US alone, at least 2 million people become infected with bacteria that are resistant to antibiotics and at least 23,000 people die each year as a direct result of those infections.39 In response to the worldwide concern, the US Centers for Medicare & Medicaid Services (CMS) recently released a proposed rule change to its Conditions of Participation which would, among other changes, require hospitals to implement antibiotic stewardship programs in order to participate in Medicare and Medicaid programs. Antibiotic stewardship includes improvement of patient outcomes by adoption of processes and procedures that reduces the incidence of infections with particular attention to pneumonia such as preventative treatment care. As a secondary benefit to the cost benefit results of this study, a significant reduction in the need for antibiotics in bronchiectasis patients by the use of SmartVest may have even greater benefits than decreasing cost. An available treatment that could significantly reduce respiratory infections and thus the need for antibiotics fits well within hospital’s infection control programs.
In summary, the clinical effectiveness of HFCWO airway clearance demonstrated by SmartVest in patients with COPD36 and those with bronchiectasis,25 and the significant reduction in antibiotic, ED and hospitalization costs observed in this study supports the cost benefit of SmartVest use and argues for insurance coverage of SmartVest by payers. Furthermore, secondary benefits such as the potential to reduce hospital readmissions and the potential to impact in deterring antibiotic resistance may have even greater benefits than decreasing cost.
Acknowledgements
The authors would like to thank Elizabeth Goodwin PhD for editorial support.
References
- King P. Pathogenesis of bronchiectasis. Paediatr Respir Rev 2011;12(2):104-110. 10.1016/j.prrv.2010.10.011
- Braverman J, Miller H. High-frequency chest compression: a practical therapy for patients with bronchiectasis. Respiratory Therapy 2008;3(1):22-26.
- Patel IS, Vlahos I, Wilkinson TM, et al. Bronchiectasis, exacerbation indices, and inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2004;170(4):400-407. 10.1164/rccm.200305-648OC
- Gono H, Fujimoto K, Kawakami S, et al. Evaluation of airway wall thickness and air trapping by HRCT in asymptomatic asthma. Eur Respir J 2003;22(6):965-971.
- Weycker D, Edelsberg J, Oster G, et al. Prevalence and economic burden of bronchiectasis. Clinical Pulmonary Medicine 2005;12(4):205-209.
- Seitz AE, Olivier KN, Adjemian J, et al. Trends in bronchiectasis among medicare beneficiaries in the United States, 2000 to 2007. CHEST Journal 2012;142(2):432-439.
- Seitz AE, Olivier KN, Steiner CA, et al. Trends and burden of bronchiectasis-associated hospitalizations in the United States, 1993-2006. Chest 2010;138(4):944-949. 10.1378/chest.10-0099
- Seitz AE, Olivier KN, Steiner CA, et al. Trends and burden of bronchiectasis-associated hospitalizations in the United States, 1993-2006. CHEST Journal 2010;138(4):944-949.
- Weycker D, Edelsberg J, Oster G, et al. Prevalence and economic burden of bronchiectasis. Clinical Pulmonary Medicine 2005;12:205-209.
- Fibrosis. AoCPiC. Standards of care and good clinical practice for the physiotherapy management ff cystic fibrosis 2011 [cited 2016 January 4]. Available from: https://www. cysticfibrosis.org.uk/media/82076/CD Standards of Care Physio Jun 11.pdf.
- O’Donnell AE. Bronchiectasis. Chest 2008;134(4):815-823. 10.1378/chest.08-0776
- Barker AF. Bronchiectasis. N Engl J Med 2002;346(18):1383-1393. 10.1056/NEJMra012519
- McCool FD, Rosen MJ. Nonpharmacologic airway clearance therapies: ACCP evidence-based clinical practice guidelines. Chest 2006;129(1 Suppl):250S-259S. 10.1378/chest.129.1 suppl.250S
- Salathe M, O’Riordan TG, Wanner A. Treatment of mucociliary dysfunction. Chest 1996;110(4):1048-1057.
- Arens R, Gozal D, Omlin KJ, et al. Comparison of high frequency chest compression and conventional chest physiotherapy in hospitalized patients with cystic fibrosis. Am J Respir Crit Care Med 1994;150(4):1154-1157. 10.1164/ajrccm.150.4.7921452
- Fink JB, Mahlmeister MJ. High-frequency oscillation of the airway and chest wall. Respir Care 2002;47(7):797-807.
- Hansen LG, Warwick WJ. High-frequency chest compression system to aid in clearance of mucus from the lung. Biomed Instrum Technol 1990;24(4):289-294.
- Lange DJ, Lechtzin N, Davey C, et al. High-frequency chest wall oscillation in ALS: an exploratory randomized, controlled trial. Neurology 2006;67(6):991-997. 10.1212/01. wnl.0000237439.78935.46
- Warwick WJ, Hansen LG. The long-term effect of high-frequency chest compression therapy on pulmonary complications of cystic fibrosis. Pediatr Pulmonol 1991;11(3):265-271.
- Kluft J, Beker L, Castagnino M, et al. A comparison of bronchial drainage treatments in cystic fibrosis. Pediatr Pulmonol 1996;22(4):271-274. 10.1002/(SICI)1099-0496(199610)22:4<271::AID-PPUL7>3.0.CO;2-P
- Scherer TA, Barandun J, Martinez E, et al. Effect of high-frequency oral airway and chest wall oscillation and conventional chest physical therapy on expectoration in patients with stable cystic fibrosis. Chest 1998;113(4):1019-1027.
- Nicolini A, Cardini F, Landucci N, et al. Effectiveness of treatment with high-frequency chest wall oscillation in patients with bronchiectasis. BMC Pulm Med 2013;13:21. 10.1186/1471-2466-13-21
- Osman LP, Roughton M, Hodson ME, et al. Short-term comparative study of high frequency chest wall oscillation and European airway clearance techniques in patients with cystic fibrosis. Thorax 2010;65(3):196-200. 10.1136/
thx.2008.111492 - Chatburn RL. High-frequency assisted airway clearance. Respir Care 2007;52(9):1224-1235; discussion 1235-1227.
- Sievert CE, Beaner CA, Sievert CP. Using High Frequency Chest Wall Oscillation in a Bronchiectasis Patient Population: An Outcomes-Based Case Review. Respiratory Therapy Journal 2016;11(4):34-38.
- HCUP. Overview of the Nationwide Emergency Department Sample (NEDS) 2016 [cited 2016 September 9]. Available from: https://www.hcup-us.ahrq.gov/nedsoverview.jsp.
- Overview of the national (nationwide) inpatient samples [cited 2016 September 9]. Available from: https://www.hcup-us.ahrq.gov/nisoverview.jsp.
- Pfuntner A, Wier LM, Steiner C. Costs for Hospital Stays in the United States, 2010: Statistical Brief #146. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD): Agency for Healthcare Research and Quality (US); 2006.
- Stein PD, Matta F. Costs of emergency department visits and hospitalizations for pulmonary arterial hypertension.
J Epidemiol Public Health Rev 2016;1(3). doi http://dx.doi. org/10.16966/2471-8211.120 - Kangovi S, Grande D. Transitional care management reimbursement to reduce COPD readmission. Chest
2014;145(1):149-155. 10.1378/chest.13-0787 - Ford ES. Hospital discharges, readmissions, and ED visits for COPD or bronchiectasis among US adults: findings
from the nationwide inpatient sample 2001-2012 and Nationwide Emergency Department Sample 2006-2011. Chest 2015;147(4):989-998. 10.1378/chest.14-2146 - Sharma G, Kuo YF, Freeman JL, et al. Outpatient follow-up visit and 30-day emergency department visit and readmission in patients hospitalized for chronic obstructive pulmonary disease. Arch Intern Med 2010;170(18):1664-1670. 10.1001/archinternmed.2010.345
- Lainscak M, Kadivec S, Kosnik M, et al. Discharge coordinator intervention prevents hospitalizations in patients with COPD: a randomized controlled trial. J Am Med Dir Assoc 2013;14(6):450 e451-456. 10.1016/j.jamda.2013.03.003
- Revitt O, Sewell L, Morgan MD, et al. Short outpatient pulmonary rehabilitation programme reduces readmission following a hospitalization for an exacerbation of chronic obstructive pulmonary disease. Respirology 2013;18(7):1063-1068. 10.1111/resp.12141
- Milne RJ, Hockey H, Rea H. Long-term air humidification therapy is cost-effective for patients with moderate or severe chronic obstructive pulmonary disease or bronchiectasis. Value Health 2014;17(4):320-327. 10.1016/j.jval.2014.01.007
- Chakravorty I, Chahal K, Austin G. A pilot study of the impact of high-frequency chest wall oscillation in chronic obstructive pulmonary disease patients with mucus hypersecretion. Int J Chron Obstruct Pulmon Dis 2011;6:693-699. 10.2147/COPD. S22896.
- Martınez-Garcıa MA, et al. Prognostic Value of Bronchiectasis in Patients with Moderate-to-Severe Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med 2013; Vol 187, Iss. 8, pp 823–831.
- O’Donnell AE. Bronchiectasis. Chest 2008;134:815–82.
- Centers for Disease Control and Prevention – Antibiotic / Antimicrobial Resistance website https://www.cdc.gov/drugresistance/
