Abstract
Background: Several recent studies have shown a significant reduction in exacerbation rates in non-cystic fibrosis bronchiectasis (NCFB) patients who received high frequency chest wall oscillation (HFCWO) as an airway clearance therapy. Other studies have reported significant improvements in pulmonary function and quality of life in NCFB patients using HFCWO therapy. However, studies that evaluate the effectiveness of long-term HFCWO treatment in NCFB populations are more limited. The aim of this study was to investigate the effect of long-term HFCWO therapy on exacerbation rates of NCFB patients.
Methods: Thirty-nine patients with a confirmed bronchiectasis diagnosis, who were known to be compliant with their
physicians prescribed HFCWO treatment regimen and meeting the inclusion/exclusion criteria, were enrolled in the study. The SmartVest Airway Clearance System (Electromed, New Prague, MN USA) was used by all patients. Exacerbations were defined as bronchiectasis-related antibiotic prescriptions, emergency department (ED) visits and hospitalization admissions. Bronchiectasis exacerbations were recorded by reviewing individual patient’s medical records for one year prior to initiating HFCWO therapy and by repeated phone interview for 2.5 years after starting treatment. Each patient served as their own control. Exacerbations recorded in the first year prior to starting HFCWO treatment were compared to the 2.5 years after starting HFCWO treatment using descriptive statistics. P values were calculated by paired t-test.
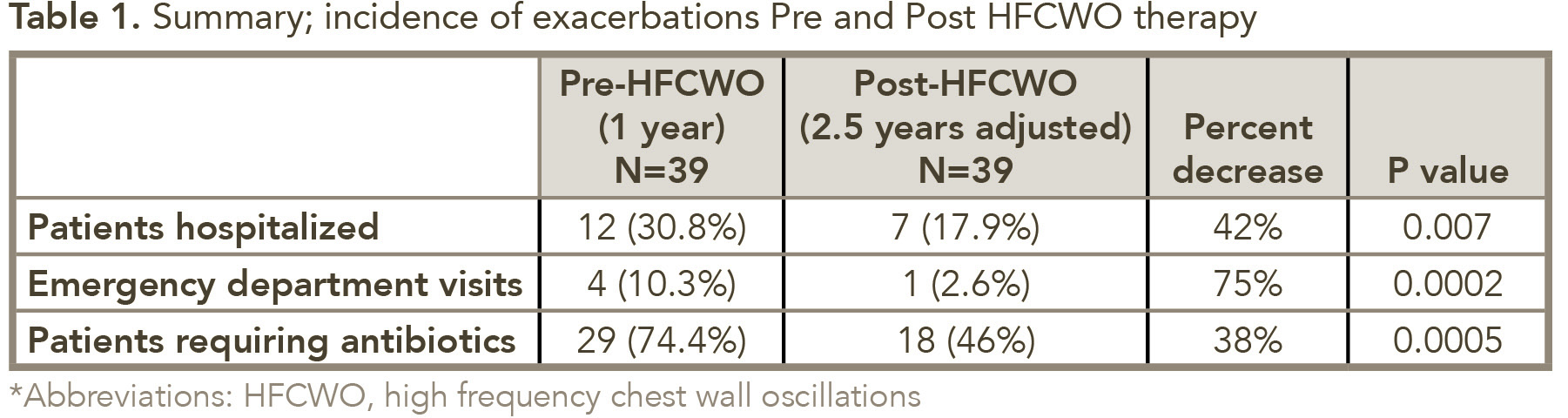
Results: Bronchiectasis-related exacerbations were significantly reduced with HFCWO therapy; the incidence of hospitalizations was decreased by 42% (P=0.007), ED visits by 75% (P=0.008) and antibiotic prescriptions by 38% (P=0.0005). Sixty-eight percent of study participants reported, at will, a significant improvement in their quality of life and a reduction in the severity of their exacerbations.
Conclusions: Previous reports of significant decreases in exacerbation rates in short-term (1 year) HFCWO studies were reproduced with statistical significance in this longer-term (2.5 year) study. Longitudinal evidence, as demonstrated by this study, further supports using HFCWO in the treatment of NCFB patients to improve clinical outcomes and enhance quality of life.
Keywords: outcome, bronchiectasis, high frequency chest wall oscillation, HFCWO, exacerbation, quality of life
Introduction
Bronchiectasis is a chronic lung disease characterized by the presence of irreversible dilation and destruction of the bronchi due to chronic inflammation and recurrent infection.1,2 The overall prevalence of non-cystic fibrosis bronchiectasis (NCFB) is about 52 per 100,000 adults and the mean age at diagnosis is 61 years.3 The incidence of hospitalization due to NCFB is rising and is markedly increased for patients ≥50 years.3,4 In 2005, the estimated costs for NCFB was $630 million annually in the United States.3,5 The same study found that patients with NCFB averaged 2.0 additional days in the hospital, had 6.1 additional outpatient encounters, 27.2 more days of antibiotic therapy, and total excess medical expenditure of $5,681 (USD).3,5
Bronchiectasis is characterized by a repetitive vicious cycle of bronchial inflammation, tissue destruction and impaired mucus clearance resulting in bacterial infection that can permanently damage the respiratory epithelium causing a significant reduction in pulmonary function, greater dyspnea, increased hospitalizations, more antibiotic use and increased morbidity and mortality4,6-9 People with NCFB commonly experience chronic cough and excessive sputum production. Diminished mucus clearance likely contributes to the initiation, progression and chronicity of NCFB.10 The accumulation of mucus can lead to infection and inflammation by providing an environment for excessive microbial growth and colonization.11 High levels of mucus in the airways are reportedly associated with increased bacterial load; in patients with NCFB, up to 64% are chronically infected with pathogenic bacteria.12 Consequently, diminished mucus clearance can increase the risk of bronchiectasis-related exacerbations, which is directly associated with increased healthcare utilization and costs (hospitalizations, emergency department (ED) visits, antibiotics, and steroid use).13,14
The goals of bronchiectasis treatment are to minimize the number of exacerbations and improve a patient’s quality of life by mobilizing airway secretions to enhance ventilation and reduce infection.15,16 It is conjectured that efficient removal of retained secretions through airway clearance techniques breaks the viscous cycle of inflammation → structural tissue damage → mucus retention → infection → repeat and thereby slowing the rate of progressive respiratory deterioration.3,17
High-frequency chest wall oscillation (HFCWO) is an airway clearance method that creates high velocity, low amplitude oscillatory airflows through a pneumatic vest worn over the thorax, and is used for enhancing airway mucus clearance in several different diseases including cystic fibrosis, bronchiectasis, asthma, COPD, and a number of neuromuscular disorders.18-21 A Cochrane review found HFCWO significantly improved health-related quality of life, lung function, ease of sputum expectoration, and reduced dyspnea and cough in patients with diminished mucus clearance.22 HFCWO is approved by the FDA to “improve bronchial drainage and enhance mucus clearance in those patients who are mucociliary compromised.” In addition, HFCWO is recommended by the American College of Chest Physicians (ACCP) as preventative treatment in patients with cystic fibrosis who have a similar viscous cycle of disease.15
A limited number of studies have evaluated the impact of HFCWO treatment outcomes in patients with NCFB.14,23 A case review study evaluated the effectiveness of the SmartVest Airway Clearance HFCWO System (Electromed, New Prague, MN, USA) on exacerbation-related healthcare utilization and medication in subjects with NCFB after one year of therapy in a “real-world setting” without patient or treatment setting stratification.14 The study found that one year of SmartVest therapy significantly reduced the incidence of bronchiectasis-related hospitalizations and ED visits. SmartVest was also associated with a significant reduction in the use of antibiotics and steroids. Here, we report longer-term findings using the same bronchiectasis patient registry, which include results after another 1.5 years of therapy to gain further insight into the effectiveness of long-term HFCWO therapy in reducing the incidence of bronchiectasis-related exacerbations.
Methods
This comparative, observational, retrospective, case review study included subjects with a radiographically confirmed diagnosis of NCFB who were being treated with HFCWO therapy. The study was performed in accordance with the Declaration of Helsinki and the Western Institutional Review Board which waved the necessity for review of the protocol and obtaining written informed consent from the subjects. However, all subjects were verbally asked for study participation prior to enrollment. All subjects had signed a HIPAA privacy agreement prior to the release of their medical records to the study Sponsor.
Study subjects
Included subjects had confirmed diagnosis of NCFB and had been using SmartVest for ≥2.5 years. Eligible patients also had medical records available for one year prior and 2.5 after start of SmartVest therapy and were compliant with their physician prescribed treatment regimen. Patients who had died or who were unable to be contacted by phone were excluded from the study. Compliance was determined by patient phone interviews, returned compliance report, and/or recorded clock hours in the SmartVest Airway Clearance generator.
Study design
A detailed description of the study methods has been previously published (see Sievert et al. [2016]).14 In brief, the rate of bronchiectasis exacerbations for one year prior to the use of HFCWO were compared the exacerbation rate after 2.5 years of HFCWO therapy. Each patient served as their own control. Bronchiectasis-related exacerbations were defined as hospitalization admissions, ED visits, and antibiotic prescriptions. Exacerbations were ascertained by review of the subject’s medical records for the one year prior to therapy and by repeated phone interview for the 2.5-year follow-up. Quality of life (QoL) was verbally reported at-will by the patient.
All data were summarized descriptively. The 2.5-year data were adjusted for comparison with the one-year data. A P-value of 0.05 or greater, as determined by the paired t-test, was considered significant.
Results
The initial study for evaluating exacerbation frequency following 1-year of HFCWO therapy included 59 patients; 39 of those were available for the additional 1.5-year follow-up. For these 39 patients, the use of HFCWO was associated with a reduction in the incidence of bronchiectasis-related exacerbations; after 2.5 years of HFCWO therapy the incidence of hospitalization decreased by 42% (P = 0.007), ED visits by 75% (P = 0.0002), and antibiotic use by 38% (P = 0.007) (see Table 1). Sixty-eight percent of the study participants reported, at-will, a substantial improvement in the QoL and a reduction in the number and severity of their exacerbations.
Discussion
Bronchiectasis has been previously underdiagnosed and overlooked although it is currently experiencing a surge in clinical interest. Numerous recent journal articles have described the importance of managing bronchiectasis-related exacerbations to improve patients’ quality of life and reduce healthcare cost. The literature reports bronchiectasis patients are more likely to have:32,33,34
- More airway mucus clearance problems,
- Greater dyspnea (2nd most common symptom after productive purulent cough),
- More persistent symptoms with greater frequency (16% not recovered by 35 days),
- More pneumonia,
- Greater functional ability decline (which is an independent predictor of long-term mortality and exacerbation frequency),
- More severe exacerbations with a longer recover time (longer hospital stays for pneumonia),
- And, most alarming, a significant potential for irreversible morbidity after an exacerbation (some patients never returning to baseline).
For these reasons, managing exacerbations can be vital to the health and well-being of NCFB patients.
The pathogenesis of bronchiectasis is well described as a vicious cycle of inflammation → airway tissue destruction → diminished mucus clearance → lung infection → repeat.35 The authors go on to discuss the generally–accepted supposition of mucus “stasis” causing increased bacterial colonization and subsequent pneumonia. Hence, airway clearance has had a surge in interest clinically as a method of breaking the bronchiectasis vicious cycle.
This longitudinal study verifies that HFCWO airway clearance therapy significantly reduces bronchiectasis-related exacerbations including the need for antibiotics, ED visits and hospitalization admissions and that this effectiveness was maintained for 2.5 years after the initiation of treatment. The decrease in antibiotic use for pneumonia (ie, 41% ↓ in year 1 compared to 38% ↓ in year 2.5) was consistent and statistically comparable. Similarly, reductions in ED visits (ie, 63% ↓ in year 1 compared to 75% year ↓ in year 2.5) and hospital admissions (ie, 42% ↓ year 1 compared to 42% ↓ in year 2.5) were demonstrated by this study. Reductions in exacerbations and antibiotic use with HFCWO therapy has been found to greatly impact health-care costs; one study found the reduction in exacerbations translated into a 60% overall reduction in healthcare utilization and cost.24 These findings indicate the long-term benefit of HFCWO therapy in patients with NCFB, and supports the clinical indication of HFCWO therapy as an FDA cleared treatment to improve bronchial drainage and enhance mucus clearance in those patients who are mucociliary compromised.
A randomized study by Nicolini et al.(2013) evaluated the effectiveness of HFCWO compared with traditional techniques of chest physiotherapy (CPT) in patients with non-cystic fibrosis bronchiectasis.23 The study found that that use of HFCWO compared to CPT produced significant improvement in the inflammation parameter C-reactive protein (P<0.019), lung function (forced vital capacity [FVC] and forced expiratory volume in one second [FEV1]; P values ≤0.006), and dyspnea. Both HFCWO and CPT were associated with significant improvements in QoL.
The benefit of HFCWO for airway clearance has also been observed in the treatment of patients with chronic obstructive pulmonary disease (COPD) or asthma. A randomized, controlled, cross-over study evaluated the effectiveness of SmartVest compared with conventional treatment in patients with moderate to severe COPD and mucus hypersecretion.25 The study found that SmartVest therapy was associated with an improvement in sputum production (P = 0.06). An improvement in the St George’s Respiratory Questionnaire including five-symptom scoring (ie, rating of cough, sputum, wheeze, shortness of breath, and exercise tolerance) was also seen.25 In another randomized, multi-center, clinical trial HFCWO treatment within 24 hours of hospital admission in acute asthma or COPD resulted in significant improvement in dyspnea compared with the control (sham) group (70.8% control vs. 42.3% treatment).26
Antibiotic therapy plays a critical role in treating exacerbations of bronchiectasis once an infection has been diagnosed.8,27 The US Bronchiectasis Research Registry found that 41% of patients were treated with antibiotics during an exacerbation and 56% used suppressive antibiotics (oral or inhaled).28
The severity of bronchiectasis is associated with an increase in the use of antibiotics; one study found that radiologic severity of bronchiectasis was correlated with the number of antibiotic courses/year (P=0.002).29 However, the use of antibiotics has major public health and therapeutic challenges, including the development of antibiotic resistance, antibiotic-related diarrhea (C. difficile), and toxicity.27,30 In addition, bacterial biofilm growth can cause chronic inflammation of the airways, and makes eradication of respiratory infection by antibiotics impossible due to the inability of the drugs to penetrate bacterial biofilms.31 Antibiotic use, particularly with systemically administered antibacterials and inhaled antibacterials, can cause bronchospasm in some patients.30 It is clinically very important to consider, due to the lack of effectiveness data, that no antibiotic type or regimen is currently approved by the FDA for bronchiectasis-related exacerbations. Consequently, the Centers for Medicare & Medicaid Services (CMS) has not approved reimbursement for an antibiotic regimen which may result in significant economic burden to both the patient and the institution. Hence, a prophylactic therapy that would reduce the need for antibiotics would have great clinical utility for not only NCFB patients but the general population.
Although statistical significance was demonstrated, the current study is limited by its small sample size. While the healthcare and prescription medication use prior to SmartVest therapy was derived from the patients’ medical chart information, the data through 2.5 years following initiation of treatment was captured by repeated individual phone interview, which was dependent upon subject recall, possibly confounding the findings. No clinical measurements were assessed, for example sputum production or lung function. We also did not collect information regarding prior airway clearance therapy, hospitalization length of stay, bronchiectasis-related outpatient visits, or changes in healthcare costs after start of SmartVest therapy.
In conclusion, this longitudinal (2.5 year) study found that the use of SmartVest continued to reduce bronchiectasis-related exacerbations with statistical significance in a non-stratified NCFB population. These findings contribute to the validation of the significant clinical utility and effectiveness of HFCWO airway clearance for the reduction of bronchiectasis-related exacerbations.
References
- Barker AF. Bronchiectasis. N Engl J Med 2002;346 (18): 1383-93. 10.1056/NEJMra012519
- Habesoglu MA, Ugurlu AO, Eyuboglu FO. Clinical, radiologic, and functional evaluation of 304 patients with bronchiectasis. Ann Thorac Med 2011;6 (3): 131-6. 10.4103/1817-1737.82443
- Braverman J, Miller H. High-frequency chest compression: a practical therapy for patients with bronchiectasis. Respiratory Therapy 2008;3 (1): 22-6.
- Seitz AE, Olivier KN, Steiner CA, Montes de Oca R, et al. Trends and burden of bronchiectasis-associated hospitalizations in the United States, 1993-2006. Chest 2010;138 (4): 944-9. 10.1378/chest.10-0099
- Weycker D, Edelsberg J, Oster G, Tino G. Prevalence and economic burden of bronchiectasis. Clinical Pulmonary Medicine 2005;12: 205-9.
- King PT, Holdsworth SR, Freezer NJ, Villanueva E, et al. Outcome in adult bronchiectasis. COPD 2005;2 (1): 27-34.
- Alzeer AH, Masood M, Basha SJ, Shaik SA. Survival of bronchiectatic patients with respiratory failure in ICU. BMC Pulm Med 2007;7: 17. 10.1186/1471-2466-7-17.
- Altenburg J, Wortel K, van der Werf TS, Boersma WG. Non-cystic fibrosis bronchiectasis: clinical presentation, diagnosis and treatment, illustrated by data from a Dutch Teaching Hospital. Neth J Med 2015;73 (4): 147-54.
- Smith MP. Non-cystic fibrosis bronchiectasis. J R Coll Physicians Edinb 2011;41 (2): 132-9; quiz 9. 10.4997/ jrcpe.2011.217
- Livraghi A, Randell SH. Cystic fibrosis and other respiratory diseases of impaired mucus clearance. Toxicol Pathol 2007;35 (1): 116-29. 10.1080/01926230601060025
- Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med 2010;363 (23): 2233-47. 10.1056/NEJMra0910061
- Angrill J, Agusti C, de Celis R, Rano A, et al. Bacterial colonisation in patients with bronchiectasis: microbiological pattern and risk factors. Thorax 2002;57 (1): 15-9.
- Suresh Babu K, Kastelik J, Morjaria JB. Role of long term antibiotics in chronic respiratory diseases. Respir Med 2013;107 (6): 800-15. 10.1016/j.rmed.2013.02.009
- Sievert CE, Beaner CA, Sievert CP. Using High Frequency Chest Wall Oscillation in a Bronchiectasis Patient Population: An Outcomes-Based Case Review. Respiratory Therapy Journal 2016;11 (4): 34-8.
- Cystic Fibrosis.. Standards of care and good clinical practice for the physiotherapy management of cystic fibrosis 2011 [cited 2016 January 4]. Available from: https://www.cysticfibrosis.org.uk/media/82076/CD_Standards_of_Care_ Physio_Jun_11.pdf.
- O’Donnell AE. Bronchiectasis. Chest 2008;134 (4): 815-23. 10.1378/chest.08-0776
- McShane PJ, Naureckas ET, Tino G, Strek ME. Non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med 2013;188 (6): 647-56. 10.1164/rccm.201303-0411CI
- Arens R, Gozal D, Omlin KJ, Vega J, et al. Comparison of high frequency chest compression and conventional chest physiotherapy in hospitalized patients with cystic fibrosis. Am J Respir Crit Care Med 1994;150 (4): 1154-7. 10.1164/ ajrccm.150.4.7921452
- Fink JB, Mahlmeister MJ. High-frequency oscillation of the airway and chest wall. Respir Care 2002;47 (7): 797-807.
- Hansen LG, Warwick WJ. High-frequency chest compression system to aid in clearance of mucus from the lung. Biomed Instrum Technol 1990;24 (4): 289-94.
- Lange DJ, Lechtzin N, Davey C, David W, et al. High-frequency chest wall oscillation in ALS: an exploratory randomized, controlled trial. Neurology 2006;67 (6): 991-7. 10.1212/01.wnl.0000237439.78935.46
- Lee AL, Burge AT, Holland AE. Airway clearance techniques for bronchiectasis. Cochrane Database Syst Rev 2015(11): Cd008351. 10.1002/14651858.CD008351.pub3
- Nicolini A, Cardini F, Landucci N, Lanata S, et al. Effectiveness of treatment with high-frequency chest wall oscillation in patients with bronchiectasis. BMC Pulm Med 2013;13: 21. 10.1186/1471-2466-13-21
- Sievert CE, Beaner CA. Cost-Effective Analysis of Using High Frequency Chest Wall Oscillation (HFCWO) in Patients with Non-Cystic Fibrosis Bronchiectasis. Respir Therapy 2017;12:45-9.
- Chakravorty I, Chahal K, Austin G. A pilot study of the impact of high-frequency chest wall oscillation in chronic obstructive pulmonary disease patients with mucus hypersecretion. Int J Chron Obstruct Pulmon Dis 2011;6: 693-9. 10.2147/COPD.S22896
- Mahajan AK, Diette GB, Hatipoglu U, Bilderback A, et al. High frequency chest wall oscillation for asthma and chronic obstructive pulmonary disease exacerbations: a randomized sham-controlled clinical trial. Respir Res 2011;12: 120. 10.1186/1465-9921-12-120
- Liapikou A, Torres A. The clinical management of lower respiratory tract infections. Expert Rev Respir Med 2016: 1-12. 10.1586/17476348.2016.1156537
- Aksamit TR, O’Donnell AE, Barker A, Olivier KN, et al. Adult Patients With Bronchiectasis: A First Look at the US Bronchiectasis Research Registry. Chest 2017;151 (5): 982-92. 10.1016/j.chest.2016.10.055
- Dimakou K, Gousiou A, Toumbis M, Kaponi M, et al. Investigation of bronchiectasis in severe uncontrolled asthma. Clin Respir J 2017. 10.1111/crj.12653
- Wilson R, Welte T, Polverino E, De Soyza A, et al. Ciprofloxacin dry powder for inhalation in non-cystic fibrosis bronchiectasis: a phase II randomised study. Eur Respir J 2013;41 (5): 1107-15. 10.1183/09031936.00071312
- Ciofu O, Tolker-Nielsen T, Jensen PO, Wang H, et al. Antimicrobial resistance, respiratory tract infections and role of biofilms in lung infections in cystic fibrosis patients. Adv Drug Deliv Rev 2015;85: 7-23. 10.1016/j.addr.2014.11.017.
- Brill SE, Patel ARC, Singh R, Mackay AJ, Brown JS, Hurst JR. Lung function, symptoms and inflammation during exacerbations of non-cystic fibrosis bronchiectasis: a prospective observational cohort study. Respiratory Research 2015; 16:16.
- Chalmers JD, Restrepo MI. Bronchiectasis Management: The State of the Union. Chest 2017 Dec;152(6):1097-1099.
- Chalmers JD, Goeminne P, Aliberti S, McDonnell MJ, Lonni S, Davidson J, Poppelwell L, Salih W, Pesci A, Dupont LJ, Fardon TC, De Soyza A, Hill AT. The bronchiectasis severity index. An international derivation and validation study. Am J Respir Crit Care Med. 2014 Mar 1;189(5):576-85.
- McShane PJ, Naureckas ET, Tino G, Strek ME. Non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med. 2013 Sep 15;188(6):647-56.
